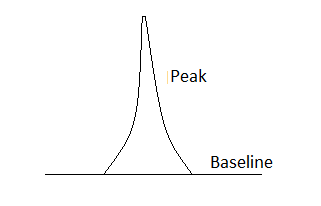
Definition of Peak:
The peak is the area of the chromatogram
which represents the detector response for the analyte eluted in chromatography
process.
The sample passes through the
stationary phase in the presence of the mobile phase. During this process, analyte
separates out from the sample mixture and produces the band separately in the
column. When that analyte detect by the detector in the form of electrical response
and it converts into the computerized data in the form of the peak of the specified analyte.
Every single analyte have a different
peak. If separation of the analyte is not proper the unresolved peak is
obtained due to a mixture of one or more analyte into the peak.
Ideal chromatography is known as the Gaussian
peak (Symmetrical Peak). Following are the characteristic of the Gaussian Peak:
·
Should be symmetric
·
Have flat base line
·
Should be sharp
Diagram of the peak:
Peak Area:
The area under the curve is known as the peak area. By using the
area the calculation of the area percentage will be done.
Peak Height:
The distance from base line to the peak apex is known as the Peak
height. The height of the peak is in proportion to quantity to the analyte
present in the sample.
Peak Overlap:
When two peaks are wide and overlap to each other it is known as
peak overlap. Proper resolution is not achieved due to the peak overlap.
Separation will not found proper.
Peak Broadening:
When peak width is increased the phenomenon is known as the Peak broadening. Broadening of the peak is due to the
longitudinal diffusion, eddy diffusion, or mass transport broadening in the
stationary phase and the dead volume.
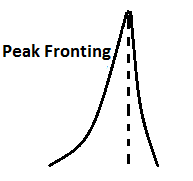
Asymmetric peak:
Asymmetrical
peak is either front or tail. Peak tailing and peak broadening is easily
understanding by the following images.

No comments:
Post a Comment